Abstract
Backrground: Several randomized controlled clinical trials have demonstrated improved outcomes for newly diagnosed multiple myeloma (NDMM) patients who were treated with lenalidomide as maintenance therapy after autologous stem cell transplant (ASCT). Proteasome inhibitors have demonstrated clinical benefit in myeloma patients when used as part of induction and maintenance regimens, and the combination of proteasome inhibitors and lenalidomide in induction regimens has produced strong clinical responses. In this study, the addition of ixazomib to lenalidomide maintenance post-ASCT in NDMM patients was evaluated.
Methods: Patients (n=64) were started on maintenance therapy with lenalidomide and ixazomib within 60-180 days of stem cell infusion. Each cycle was defined as 28 days with lenalidomide starting at 10 mg/day orally for 28 days with the option to increase the dose to 15 mg after 3 cycles. Ixazomib was provided at 3 mg (n=48 patients) or 4 mg (n=16 patients) orally on days 1, 8, and 15 of each 28-day cycle. However, ixazomib dose was reduced to 3 mg in all patients based on toxicity observed in other clinical trials of ixazomib at that time. The primary endpoint measured was progression-free survival (PFS), which was defined as the time between ASCT and disease progression or death, whichever occurred first.
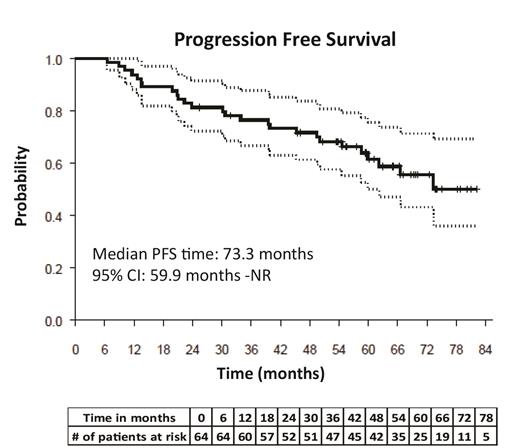
Results: A total of 64 patients were enrolled on this study between December 4, 2012, and May 13, 2015. Of these patients, 41 (64.06%) were 60 years of age or older and 42 (65.63%) were male. Fourteen patients had high-risk cytogenetic features (+1q21, Del17p, t(14:16), t(4:14)), 50 patients had standard cytogenetic risk features (t(11:14), t(6:14), hyperdiploidy, normal) and 9 patients had International Staging System stage 3 disease. Median PFS (mPFS) for all patients was 73.3 months and has not been reached for those with ISS stage 1 disease. mPFS for ISS Stage 3 disease and high-risk cytogenetic subgroups was 33.8 and 25.4 months, respectively. Twenty-two patients had progressive disease, while 21 patients continue to receive dual maintenance. Response rates deepened over time from baseline post-ASCT for 39 patients. The complete response (CR)/stringent CR rate was 42.9% and median overall survival was not reached with a median follow-up of 62 months (range 25.4 - 82.1 months). Thirty-one patients (48%) had improvement from their baseline response after maintenance therapy: 6 patients improved from PR to VGPR; 7 from PR to stringent CR (sCR)/CR; 16 from VGPR to sCR/CR; 1 from SD to CR; and 1 patient improved from SD to VGPR. The median time to response in the 31 patients with improved response to maintenance therapy was 10.9 months (range, 0.9 to 51.3 months). Minimal residual disease (MRD) was evaluated by multicolor flow cytometry (10^-5) in 21 patients by bone marrow biopsy; 8 patients were MRD-positive. The most common grade 3/4 adverse events (AEs) included neutropenia (46.9%), leukopenia (20.3%), thrombocytopenia (15.6%), lung infections (26.6%), diarrhea and maculopapular rash (12.5% each). Secondary primary malignancies occurred in 9 patients; these included squamous cell carcinoma of the skin (n=4), basal cell carcinoma of the skin (n=1), squamous cell carcinoma and basal cell carcinoma of the skin (n=1), hepatocellular carcinoma (n=1), melanoma (n=1) and leukemia (n=1). AEs led to dose reductions in ixazomib and lenalidomide in 20 and 31 patients, respectively. Discontinuation of ixazomib due to AEs occurred in 4 patients. Grade 1/2 neuropathy occurred in 22 patients and led to reduction or discontinuation of ixazomib in 2 patients.
Conclusion: Addition of ixazomib to lenalidomide maintenance in myeloma patients demonstrated a better than expected PFS compared with what has been reported in studies of lenalidomide alone, and was both safe and tolerable. These results indicate a significant clinical benefit, especially for standard risk patients.
Patel: Oncopeptides: Consultancy; Pfizer: Consultancy; Janssen: Consultancy, Research Funding; BMS Celgene: Consultancy, Research Funding. Shah: Karyopharm Therapeutics Inc.: Current Employment, Current equity holder in publicly-traded company. Lee: GlaxoSmithKline: Consultancy, Research Funding; Bristol Myers Squibb: Consultancy; Celgene: Consultancy; Regeneron: Research Funding; Takeda Pharmaceuticals: Consultancy, Research Funding; Oncopetides: Consultancy; Amgen: Consultancy, Research Funding; Karyopharm: Consultancy; Legend Biotech: Consultancy; Sanofi: Consultancy; Janssen: Consultancy, Research Funding; Genentech: Consultancy. Thomas: BeiGene: Membership on an entity's Board of Directors or advisory committees; BMS: Research Funding; Ascentage Pharma: Research Funding; X4 Pharma: Research Funding; Genentech: Research Funding; Acerta Pharma: Research Funding; Pharmacyclics: Membership on an entity's Board of Directors or advisory committees. Qazilbash: NexImmune: Research Funding; Angiocrine: Research Funding; Amgen: Research Funding; Bristol-Myers Squibb: Other: Advisory Board; Oncopeptides: Other: Advisory Board; Janssen: Research Funding; Biolline: Research Funding. Orlowski: Amgen, Inc., BioTheryX, Inc., Bristol-Myers Squibb, Celgene, EcoR1 Capital LLC, Genzyme, GSK Biologicals, Janssen Biotech, Karyopharm Therapeutics, Inc., Neoleukin Corporation, Oncopeptides AB, Regeneron Pharmaceuticals, Inc., Sanofi-Aventis, and Takeda P: Consultancy, Honoraria; Asylia Therapeutics, Inc., BioTheryX, Inc., and Heidelberg Pharma, AG.: Other: Laboratory research funding; CARsgen Therapeutics, Celgene, Exelixis, Janssen Biotech, Sanofi-Aventis, Takeda Pharmaceuticals North America, Inc.: Other: Clinical research funding; Asylia Therapeutics, Inc.: Current holder of individual stocks in a privately-held company, Patents & Royalties; Amgen, Inc., BioTheryX, Inc., Bristol-Myers Squibb, Celgene, Forma Therapeutics, Genzyme, GSK Biologicals, Janssen Biotech, Juno Therapeutics, Karyopharm Therapeutics, Inc., Kite Pharma, Neoleukin Corporation, Oncopeptides AB, Regeneron Pharmaceuticals, I: Membership on an entity's Board of Directors or advisory committees.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal